According To The Animation, Which Compounds Provide Electrons To The System?
17.five: Phosphorylation Mechanisms for Generating ATP
- Page ID
- 3404
Learning Objectives
- Define photophosphorylation.
- Draw substrate-level phosphorylation and proper name to energy-generating pathways in which this occurs.
- Define oxidative phosphorylation.
- Proper name the two components of a hydrogen atom.
- Describe an oxidation-reduction reaction.
- Ascertain dehydrogenation and hydrogenation.
- Land the role of the post-obit coenzymes and requite their reduced form:
- NAD+
- FAD
- NADP+
- Briefly draw proton motive forcefulness and how it develops within a prison cell.
- Describe an electron send concatenation and land its cellular function.
- Briefly describethe chemiosmotic theory of generation of ATP as a outcome of an electron ship concatenation.
- State the function of ATP synthases.
Substrate-Level Phosphorylation
Substrate-level phosphorylation is the production of ATP from ADP by a direct transfer of a high-energy phosphate group from a phosphorylated intermediate metabolic compound in an exergonic catabolic pathway every bit shown in Figure \(\PageIndex{2}\). Such intermediate compounds are sometimes chosen loftier-energy transfer compounds (HETCs) and several HETCs are found as intermediates during glycolysis and aerobic respiration .
Oxidative Phosphorylation
Oxidative phosphorylation is the production of ATP using energy derived from the transfer of electrons in an electron transport system and occurs by chemiosmosis.
To empathise oxidative phosphorylation, information technology is important to first review the hydrogen atom and the process of oxidation and reduction. An atom of hydrogen contains simply one proton (H+) and one electron (due east-). Therefore, the term proton and the term hydrogen ion (H+) are interchangeable. As well remember that electrons take stored free energy, or potential energy, ready to exercise work and when an atom or molecule loses that electron (becomes oxidized) that energy is released and able to do cellular piece of work.
Oxidation-reduction reactions are coupled chemical reactions in which one atom or molecule loses one or more electrons (oxidation ) while another atom or molecule gains those electrons (reduction ). The chemical compound that loses electrons becomes oxidized; the compound that gains those electrons becomes reduced. In covalent compounds, however, it is ordinarily easier to lose a whole hydrogen (H) cantlet - a proton and an electron - rather than just an electron. An oxidation reaction during which both a proton and an electron are lost is called dehydrogenation . A reduction reaction during which both a proton and an electron are gained is called hydrogenation .
Cells utilize specific molecules to acquit the electrons that are removed during the oxidation of an energy source. These molecules are called electron carriers and they alternately become oxidized and reduced during electron and proton transfer. These include three freely diffusible coenzymes known every bit NAD+, FAD, and NADP+. The reduced forms of these coenzymes (NADH, FADH2, and NADPH) take reducing power because their bonds incorporate a form of usable energy.
- NAD+ , or nicotinamide adenine dinucleotide, is a coenzyme that often works in conjunction with an enzyme chosen a dehydrogenase. The enzyme removes 2 hydrogen atoms (2H+ and 2e-) from its substrate. Both electrons but just one proton are accepted by the NAD+ to produce its reduced form, NADH, plus H+. NADH is used to generate proton motive force (discussed beneath) that tin drive the synthesis of ATP.
- FAD , or flavin adenine dinucleotide, is a coenzyme that besides works in conjunction with an enzyme called a dehydrogenase. The enzyme removes two hydrogen atoms (2H+ and 2e-) from its substrate. Both electrons and both protons are accepted by the FAD to produce its reduced form, FADHii. FADHii is used to generate proton motive force (discussed below) that tin drive the synthesis of ATP.
- NADP+, or nicotinamide adenine dinucleotide phosphate, is a coenzyme that uses dehydrogenase to remove two hydrogen atoms (2H+ and 2e-) from its substrate. Both electrons but only one proton are accepted by the NADP+ to produce its reduced form, NADPH, plus H+. NADPH is not used for ATP synthesis but its electrons provide the free energy for sure biosynthesis reactions such every bit ones involved in photosynthesis.
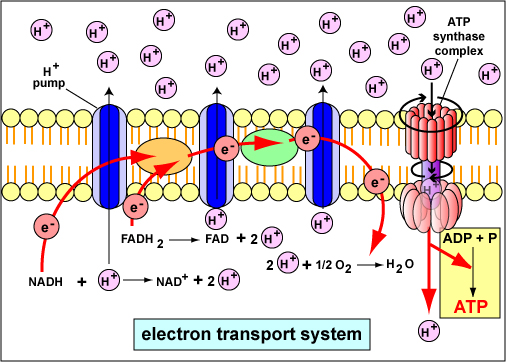
During the process of aerobic respiration, discussed in the next section, coupled oxidation-reduction reactions and electron carriers are often part of what is called an electron transport chain , a series of electron carriers that eventually transfers electrons from NADH and FADHii to oxygen. The diffusible electron carriers NADH and FADHtwo behave hydrogen atoms (protons and electrons) from substrates in exergonic catabolic pathways such as glycolysis and the citric acrid cycle to other electron carriers that are embedded in membranes. These membrane-associated electron carriers include flavoproteins, iron-sulfur proteins, quinones, and cytochromes. The last electron carrier in the electron transport chain transfers the electrons to the terminal electron acceptor, oxygen.
The chemiosmotic theory explains the functioning of electron transport chains. Co-ordinate to this theory, the transfer of electrons down an electron transport system through a series of oxidation-reduction reactions releases free energy (Figure \(\PageIndex{one}\)). This free energy allows sure carriers in the chain to transport hydrogen ions (H+ or protons) across a membrane.
Depending on the type of cell, the electron transport chain may be found in the cytoplasmic membrane, the inner membrane of mitochondria, and the inner membrane of chloroplasts.
- In prokaryotic cells, the protons are transported from the cytoplasm of the bacterium across the cytoplasmic membrane to the periplasmic space located between the cytoplasmic membrane and the cell wall.
- In eukaryotic cells, protons are transported from the matrix of the mitochondria beyond the inner mitochondrial membrane to the intermembrane infinite located betwixt the inner and outer mitochondrial membranes.
- In plant cells and the cells of algae, protons are transported from the stroma of the chloroplast across the thylakoid membrane into the interior space of the thylakoid.
As the hydrogen ions accumulate on i side of a membrane, the concentration of hydrogen ions creates an electrochemical gradient or potential difference (voltage) beyond the membrane. (The fluid on the side of the membrane where the protons accumulate acquires a positive charge; the fluid on the contrary side of the membrane is left with a negative charge.) The energized land of the membrane as a result of this charge separation is chosen proton motive force or PMF.
This proton motive force provides the free energy necessary for enzymes called ATP synthases (Figure \(\PageIndex{5}\)), also located in the membranes mentioned above, to catalyze the synthesis of ATP from ADP and phosphate. This generation of ATP occurs equally the protons cross the membrane through the ATP synthase complexes and re-enter either the bacterial cytoplasm (Figure \(\PageIndex{five}\)), the matrix of the mitochondria, or the stroma of the chloroplasts. As the protons motion down the concentration gradient through the ATP synthase, the energy released causes the rotor and rod of the ATP synthase to rotate. The mechanical free energy from this rotation is converted into chemical energy as phosphate is added to ADP to form ATP.
Proton motive force is also used to ship substances across membranes during agile transport and to rotate bacterial flagella.
At the end of the electron transport chain involved in aerobic respiration, the last electron carrier in the membrane transfers two electrons to one-half an oxygen molecule (an oxygen atom) that simultaneously combines with 2 protons from the surrounding medium to produce h2o as an end product (Figure \(\PageIndex{3}\)). The electron send bondage involved in photosynthesis ultimately transfer 2 electrons to NADP+ that simultaneously combines with 2 protons from the surrounding medium to produce NADPH.
Summary
- Photophosphorylation uses the radiant free energy of the dominicus to drive the synthesis of ATP.
- This is a process seen just in cells capable of photosynthesis.
- Substrate-level phosphorylation is the product of ATP from ADP past a direct transfer of a high-energy phosphate group from a phosphorylated intermediate metabolic compound in an exergonic catabolic pathway.
- Oxidative phosphorylation is the production of ATP using energy derived from the transfer of electrons in an electron transport system and occurs past chemiosmosis.
- An cantlet of hydrogen contains only ane proton (H+) and one electron.
- Electrons accept stored energy, or potential energy, prepare to do work. When an atom or molecule loses that electron (becomes oxidized) that energy is released and able to exercise cellular piece of work.
- Oxidation-reduction reactions are coupled chemic reactions in which ane cantlet or molecule loses one or more than electrons (oxidation) while another atom or molecule gains those electrons (reduction).
- An oxidation reaction during which both a proton and an electron are lost is chosen dehydrogenation.
- A reduction reaction during which both a proton and an electron are gained is called hydrogenation.
- Cells employ specific molecules such as NAD+, FAD, and NADP+ to acquit the electrons that are removed during the oxidation of an energy source. These molecules are called electron carriers and they alternately become oxidized and reduced during electron and proton transfer.
- Coupled oxidation-reduction reactions and electron carriers are often role of what is called an electron transport chain.
- The chemiosmotic theory explains the functioning of electron transport chains. According to this theory, the transfer of electrons down an electron transport system through a series of oxidation-reduction reactions releases energy. This energy allows certain carriers in the concatenation to transport hydrogen ions (H+ or protons) across a membrane.
- In prokaryotic cells, the protons are transported from the cytoplasm of the bacterium beyond the cytoplasmic membrane to the periplasmic infinite located betwixt the cytoplasmic membrane and the cell wall; in eukaryotic cells, protons are transported from the matrix of the mitochondria across the inner mitochondrial membrane to the intermembrane space located between the inner and outer mitochondrial membranes; in plant cells and the cells of algae, protons are transported from the stroma of the chloroplast beyond the thylakoid membrane into the interior infinite of the thylakoid.
- As the hydrogen ions accumulate on 1 side of a membrane, the concentration of hydrogen ions creates an electrochemical gradient or potential difference (voltage) across the membrane called proton motive force (PMF).
- This proton motive force provides the energy necessary for enzymes called ATP synthases to catalyze the synthesis of ATP from ADP and phosphate.
Questions
Study the material in this section and then write out the answers to these question. Do not just click on the answers and write them out. This will not test your understanding of this tutorial.
- Define photophosphorylation. (ans)
- Briefly draw the process of substrate-level phosphorylation. (ans)
- Briefly describe the procedure of oxidative phosphorylation. (ans)
- Another proper name for a hydrogen ion (H+) is: (ans)
- An cantlet or molecule gains an electron. This best describes:
- oxidation (ans)
- reduction (ans)
- dehydrogenation (ans)
- hydrogenation (ans)
- When a molecule gains electrons or both protons and electrons, we say it becomes:
- oxidized (ans)
- reduced (ans)
- Cells use specific molecules to conduct the electrons that are removed during the oxidation of an energy source. These molecules are called electron carriers and they alternately become oxidized and reduced during electron and proton transfer. Name iii freely diffusible coenzymes and give both their oxidized and reduced state. (ans)
- A coenzyme that often works in conjunction with an enzyme called a dehydrogenase. The enzyme removes two hydrogen atoms (2H+ and 2e-) from its substrate. Both electrons but only one proton are accepted to produce its reduced grade that is used to generate proton motive force for driving the synthesis of ATP. This all-time describes:
- NAD+ (ans)
- FAD (ans)
- NADP+ (ans)
- NADH + H+ is the ________________ form of NAD+. (ans)
- Describe an electron transport chain. (ans)
- Based on the chemiosmotic theory, briefly depict proton motive force and how it develops within a cell. (ans)
- Based on the chemiosmotic theory, briefly depict how proton motive force leads to the generation of ATP. (ans)
Source: https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Kaiser)/Unit_7%3A_Microbial_Genetics_and_Microbial_Metabolism/17%3A_Bacterial_Growth_and_Energy_Production/17.5%3A_Phosphorylation_Mechanisms_for_Generating_ATP
Posted by: truongweravive.blogspot.com

0 Response to "According To The Animation, Which Compounds Provide Electrons To The System?"
Post a Comment